Which Transition Emits Light With The Shortest Wavelength?
Khalid Young Dumb And Broke, Khalid - Young Dumb & Broke (Official Video), 5.68 MB, 04:08, 860,329,150, KhalidVEVO, 2017-08-01T14:00:02.000000Z, 19, Khalid – Young Dumb & Broke Lyrics | Genius Lyrics, genius.com, 1000 x 1000, jpeg, dumb broke young khalid lyrics genius, 20, khalid-young-dumb-and-broke, Kampion
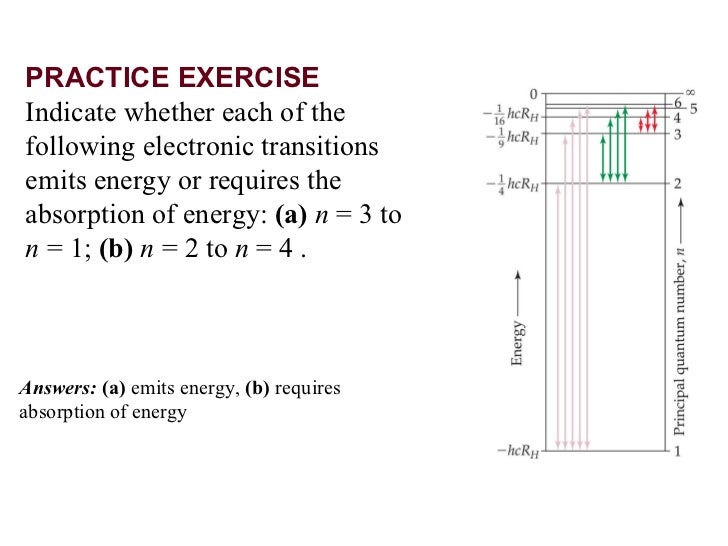
Which transition will result in emitted light with the shortest. Which transition emits light with the highest energy? Which transition emits the shortest wavelength of light? How many d orbitals exist in one energy level (n ≥.
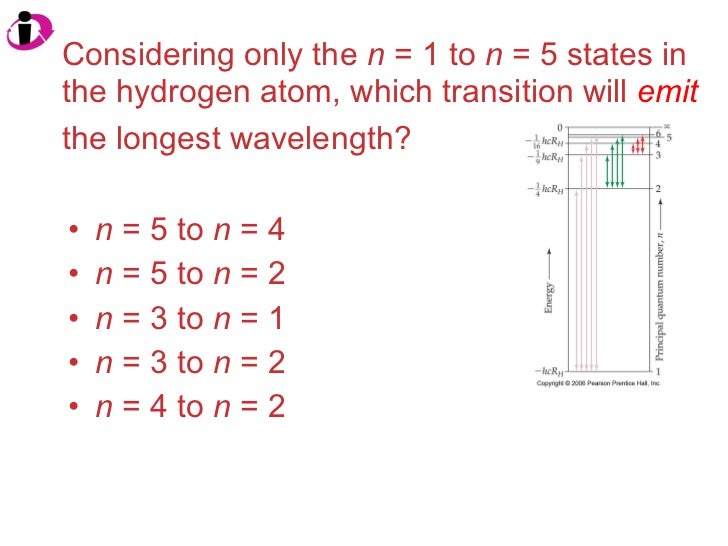
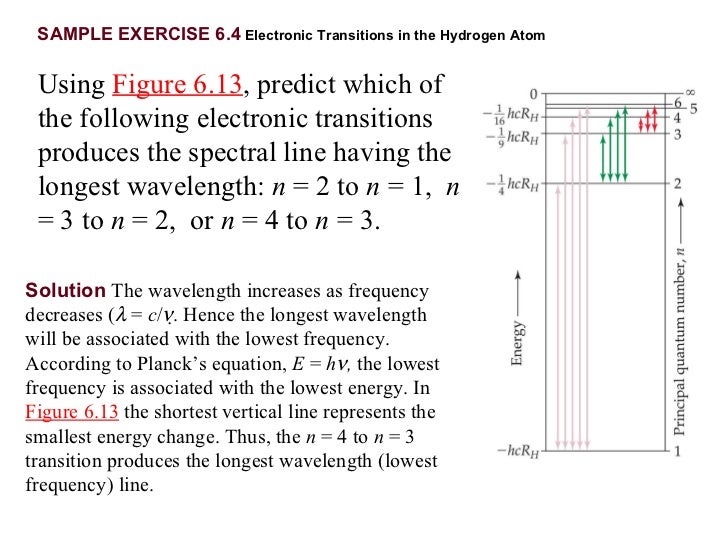
Which transition emits the shortest wavelength of light? N = 5 to n = 1. The transition that gives light the shortest wavelength is the one that produces the most energy change. Among the given transitions, the energy difference for the transition n 4 → n 1 is maximum. Higher is the energy difference, lower is the wavelength. The transition that gives light the. Correct option is b) δe= r h(n 121 − n 221) e= λhc. From the above formulae, the decreasing order of energy of transition is n= 4ton=1, n=3ton=1, n=4ton=2, n=3ton=2. This should be the.
Chapter 6 Lecture- Electrons in Atoms

Atomic Structure
An electron in the n = 7 level of the hydrogen atom relaxes to a lower

Phy 310 chapter 5
Phy 310 chapter 5
Is there something beyond gamma rays? - Quora
The Following Is A Diagram Of Energy States And Transitions In The
Phy 310 chapter 4

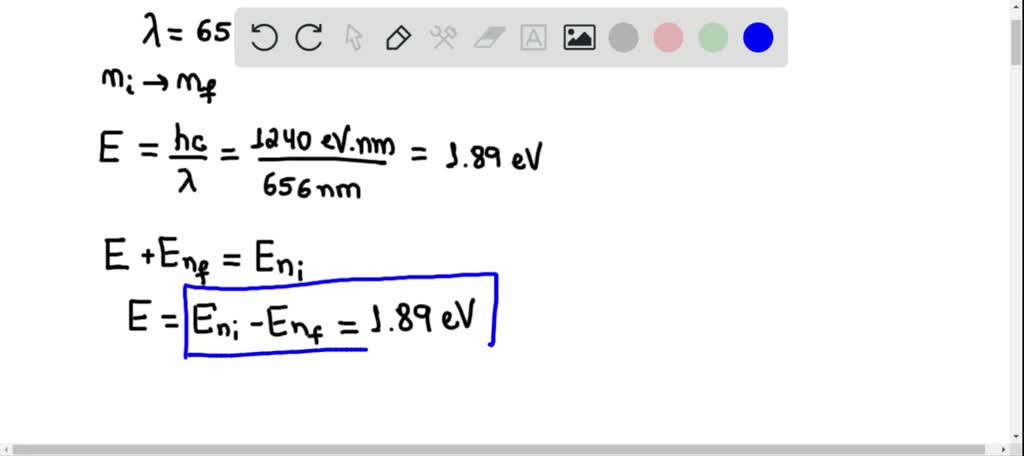
SOLVED:A hydrogen atom emits a photon of waveleng…
Hydrogen Atom: Hydrogen Atom Longest Wavelength