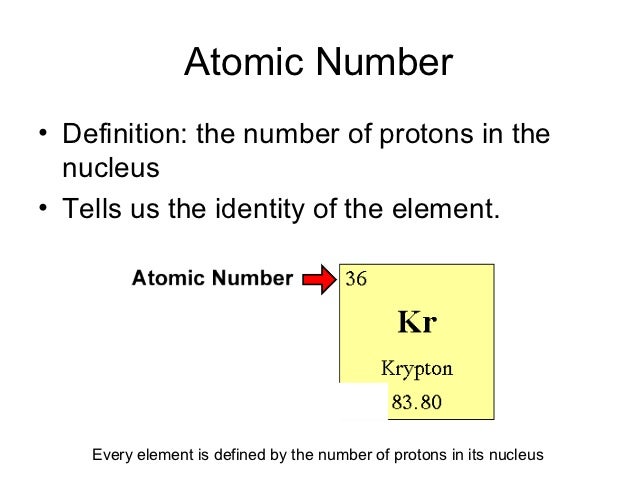
What Does The Number Next To Isotopes Signify

Khalid Young Dumb And Broke, Khalid - Young Dumb & Broke (Official Video), 5.68 MB, 04:08, 860,329,150, KhalidVEVO, 2017-08-01T14:00:02.000000Z, 19, Khalid – Young Dumb & Broke Lyrics | Genius Lyrics, genius.com, 1000 x 1000, jpeg, dumb broke young khalid lyrics genius, 20, khalid-young-dumb-and-broke, Kampion
What does the number next to the ion signify? What is the number at the end of the isotopes name? Answer expert verified the number of protons and electrons remain same for two isotopes. This means isotopes have different.
What is the number at the end of the isotopes name? Answer expert verified the number of protons and electrons remain same for. What does the number next to isotopes signify? It is an approximation of its mass number. Each isotope of an element has a different mass number. The number next to isotopes signifies the mass number of the isotope. Isotopes are versions of the same element. They have the same number of protons and electrons as the element but different mass numbers and number of. What does the number next to isotopes signify?
Atomic number, mass number, and isotopes | Chemistry | Khan Academy

Atomic structure part 1 fundamental particles

Periodic Table Numbers Meaning | Review Home Decor
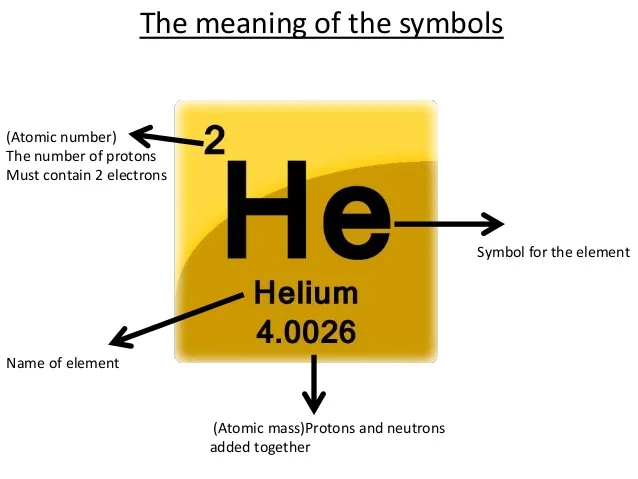
Ion & Isotope Practice Key - YouTube
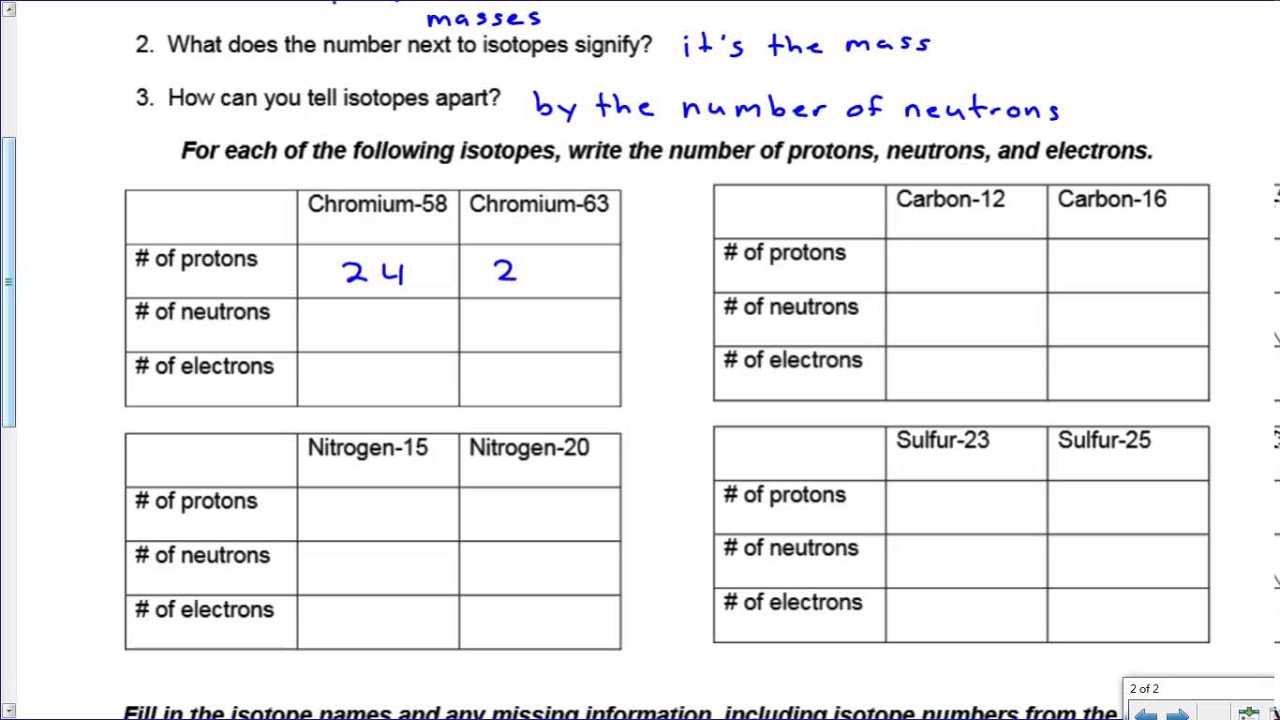
3 - Isotopes - IGCSE Chemistry

Number of isotopes in helium. 😱 How many protons electrons and neutrons
How to determine which isotope is the most abundant - YouTube

Solved: Write Isotopic Symbols In The Form X-A (e.g., C-13... | Chegg.com

Periodic table power point pres