What Is The Maximum Number Of D Orbitals That Are Possible

Khalid Young Dumb And Broke, Khalid - Young Dumb & Broke (Official Video), 5.68 MB, 04:08, 860,329,150, KhalidVEVO, 2017-08-01T14:00:02.000000Z, 19, Khalid – Young Dumb & Broke Lyrics | Genius Lyrics, genius.com, 1000 x 1000, jpeg, dumb broke young khalid lyrics genius, 20, khalid-young-dumb-and-broke, Kampion
The p orbitals line up orthogonally to each other in a dumbbell shape. In three dimensions, there are 3 orbitals possible: Each of the p orbitals can hold 2. The maximum number of electrons that a single orbital can hold is two.
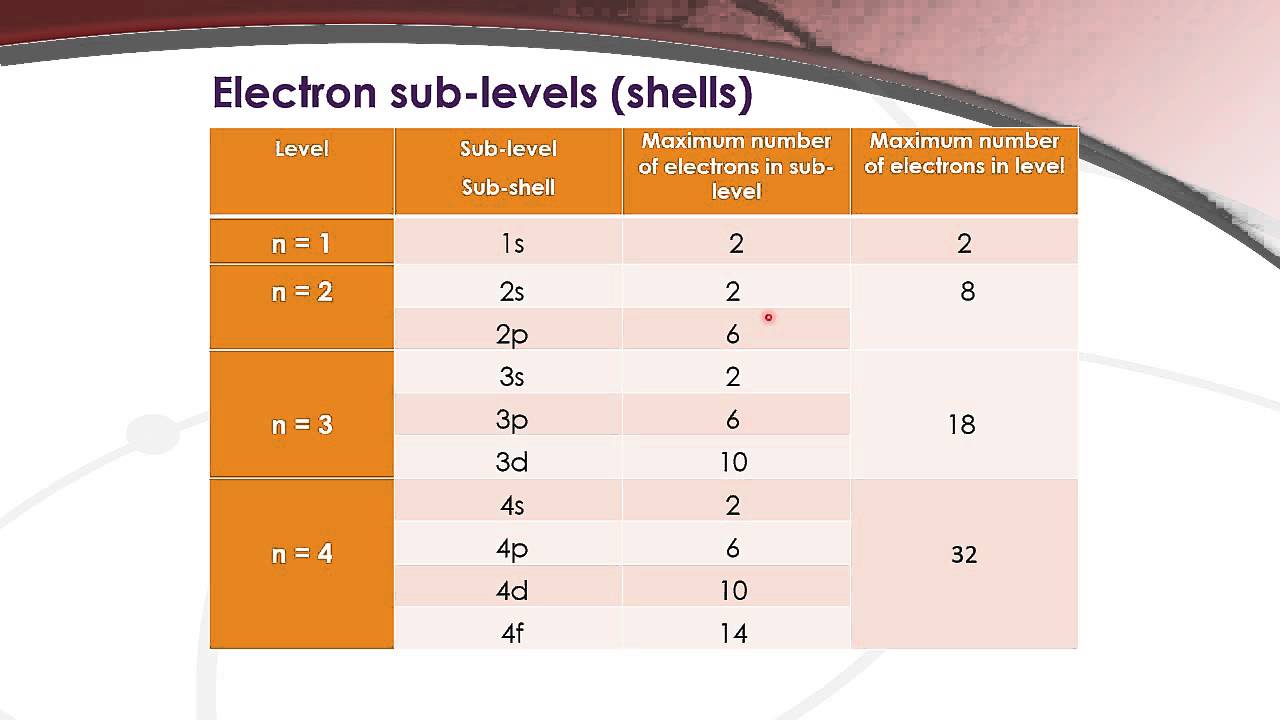
The subshells which include s, p, d, and f. What is the maximum number of orbitals that can be identified with the quantum number n=3,l=1,m=0 00:35 min 320 kbps 820. 31 kb downlod now Correct option is d) maximum number of electrons held in the d orbitals is 10. What is the maximum number of d orbital? The d sublevel has 5 orbitals so can contain 10 electrons max. How many energy levels are in the d orbital? Orbitals are possible for the following quantum numbers? N = 4, l = 2, m =. The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals, but each one.
Solved: 6. What Is The Maximum Number Of D Orbitals That A... | Chegg.com

PPT - Electron Configurations PowerPoint Presentation, free download
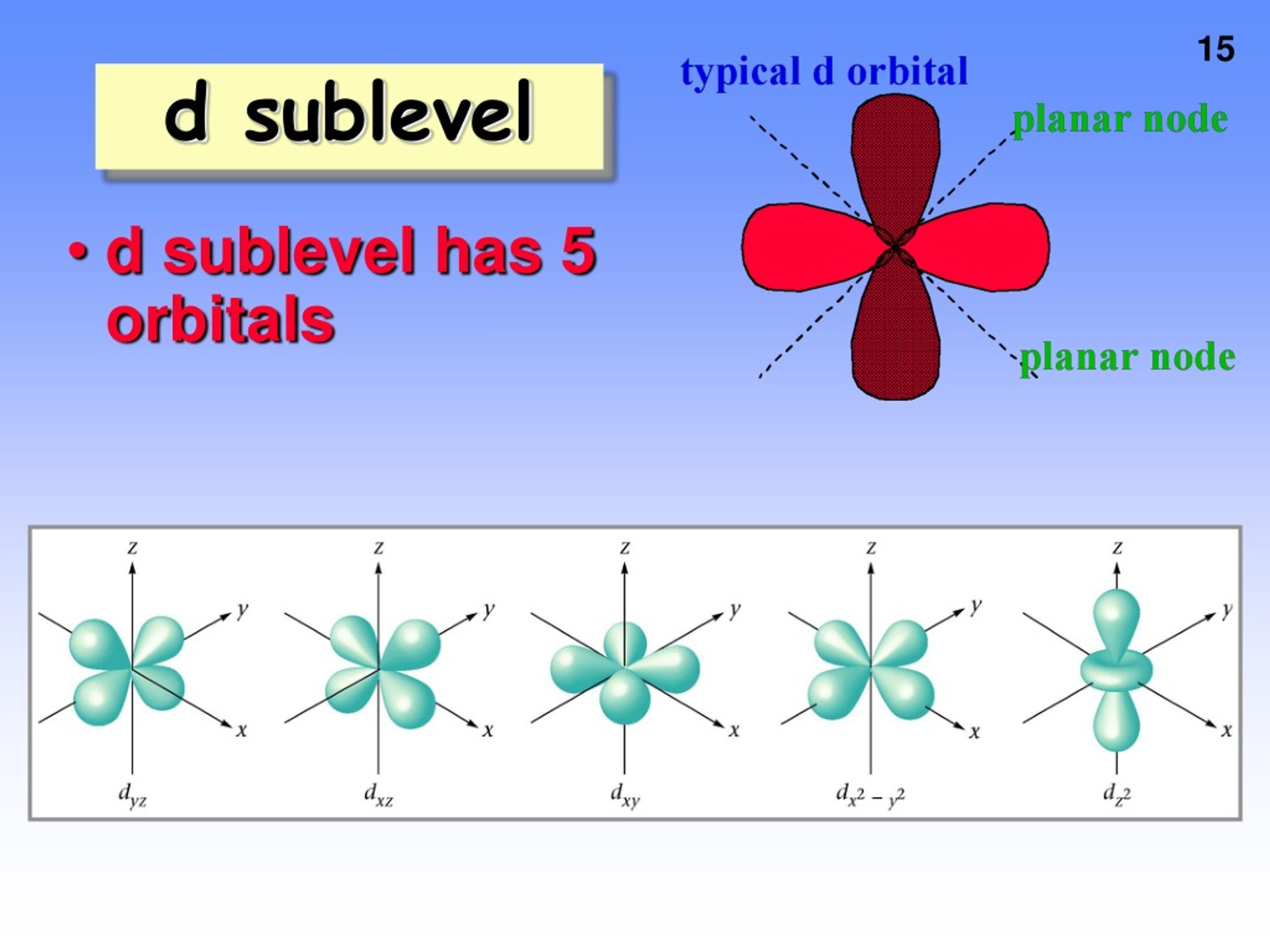
12.1.4 State the maximum number of orbitals in a given energy level
Chapter 7 notes
CHEMISTREE11: April 20 2011 - Electron Configuration

How many sub-shell are there in N shell ? How many orbitals are there

【Tips】Electron Configuration(with Video):How Do You Get the Electron

CH150: Chapter 2 – Atoms and Periodic Table – Chemistry
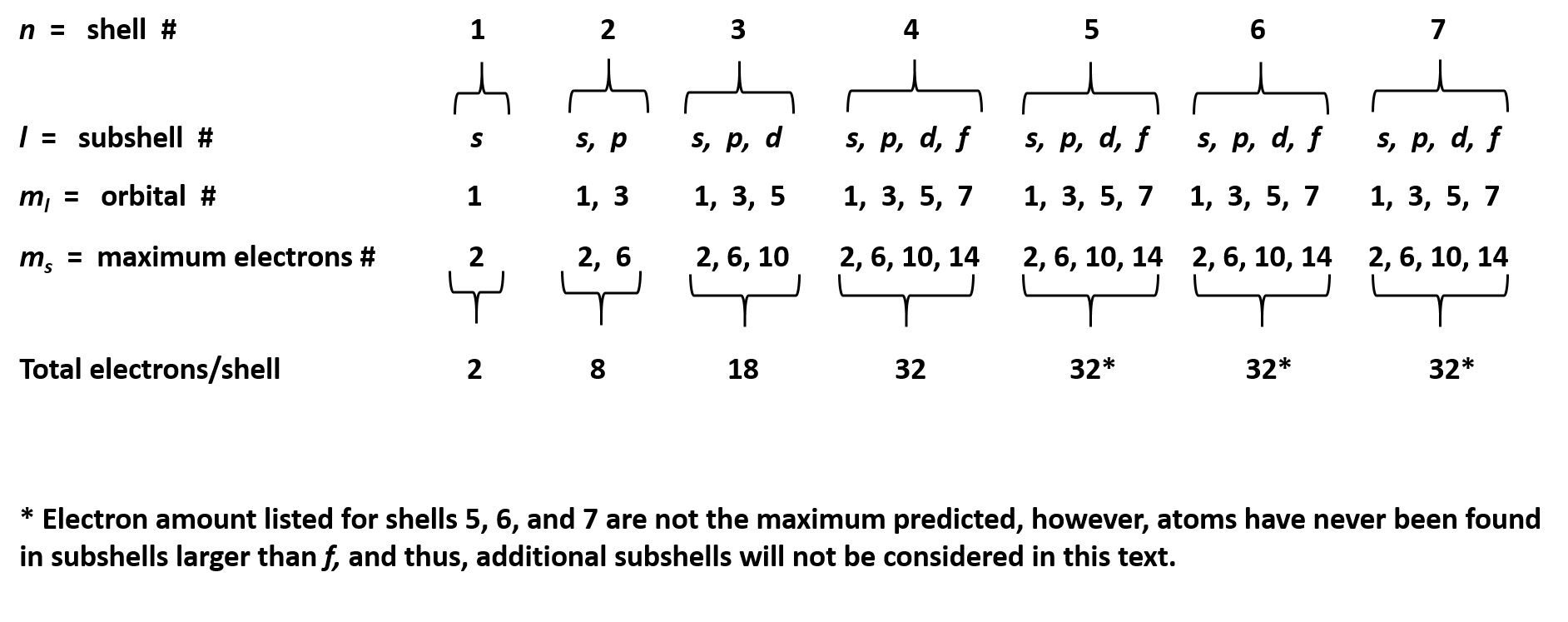
PPT - Quantum Numbers PowerPoint Presentation, free download - ID:3181772

PPT - Ch. 7 Atomic and Electronic Structure PowerPoint Presentation
